Myocarditis/heart autoimmunity
Experimental autoimmune myocarditis
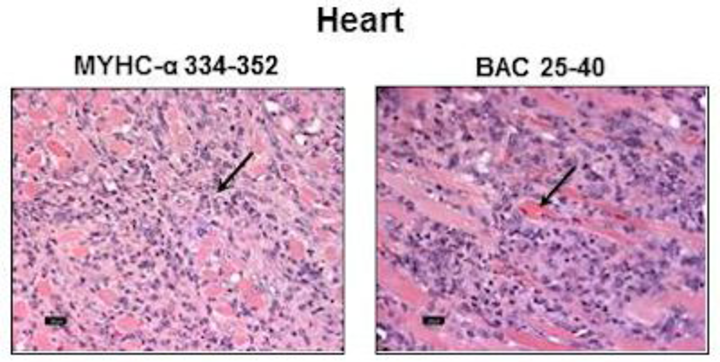
Using cardiac myosin heavy chain (Myhc)-α 334-352 as a cardiac autoantigen, we recently discovered a novel bacterium called Bacillus infantis NRRL B-14911 that has the potential to induce autoimmune myocarditis in A/J mice through antigenic mimicry. Our data support the notion that exposure to environmental microbes, which are otherwise innocuous, can predispose to the development of myocardial damage in genetically susceptible individuals. We are currently investigating the pathogenic attributes of Bacillus infantis in the mediation of infectious myocarditis.
Coxsackievirus B3 (CVB3)-induced myocarditis
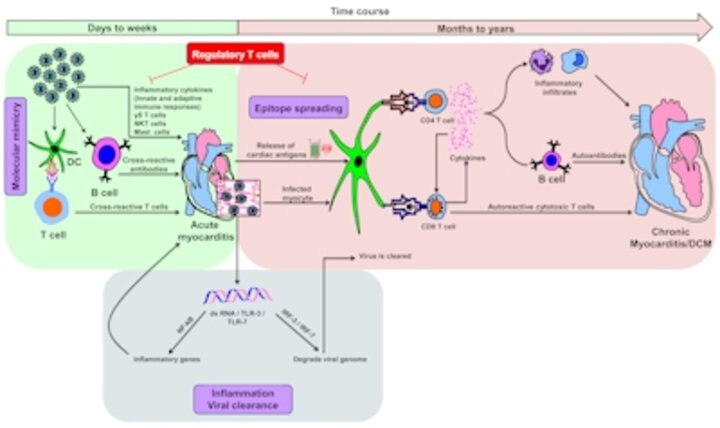
Using CVB3 as a bona fide pathogen of the cardiovascular system, we addressed the long-standing question whether chronic myocarditis that occurs in CVB3 infection is due to an autoimmune response to cardiac antigens. Using myocarditis-susceptible A/J mice, we demonstrated that animals infected with CVB3 show the generation of Myhc-α-specific CD4 T cells that can transfer the disease to naïve recipients. We suggest that pathogens like CVB3 that primarily infect hearts can lead to secondary generation of pathogenic autoimmune responses resulting from release of self-antigens due to cardiac injury. We propose that the immune-mediated damage is superimposed on the initial tissue destruction caused by the virus, but once autoimmune response sets in, the disease process becomes perpetual, leading to the development of chronic myocarditis/dilated cardiomyopathy. We are now delineating these events.
Multiple sclerosis (MS) / central nervous system (CNS) autoimmunity
Experimental autoimmune encephalomyelitis (EAE)
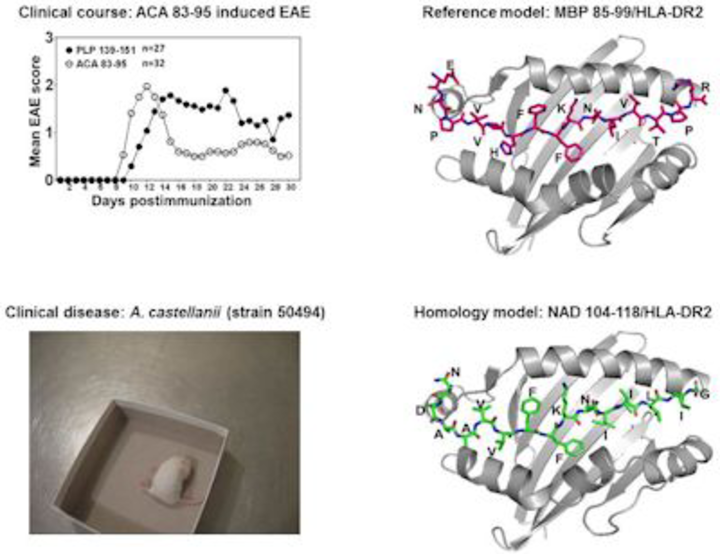
EAE models have been traditionally used to address the immune events in the pathogenesis of multiple sclerosis (MS). We focus on two models: EAE induced with myelin proteolipid protein (PLP) 139-151 and EAE induced with myelin oligodendrocyte glycoprotein (MOG) 35-55, in SJL and C57Bl/6 mice, respectively. Using PLP 139-151 as a model antigen, we discovered that Acanthamoeba castellaniicastellanii has the potential to trigger MS by generating cross-reactive T cells for two well-characterized myelin antigens, PLP 139-151 and myelin basic protein 85-99. Our preliminary results suggest that MS patients, but not patients with other neurological disorders, show evidence of Acanthamoeba-genomic material in their cerebrospinal fluids.
Role of reactive oxygen species in self-tolerance and autoimmunity
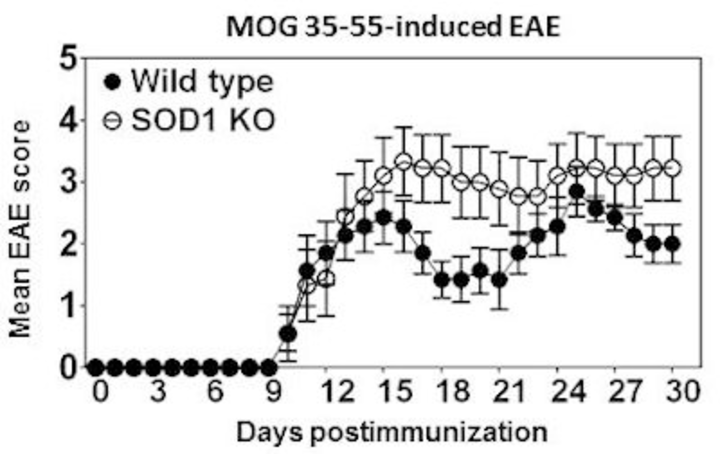
The general dogma is that increased reactive oxygen species (ROS) production is detrimental and causes tissue injuries. However, this paradigm has been recently revisited with an observation that oxidative burst in fact is beneficial in controlling autoimmune responses. To directly address the role of ROS in the mediation of CNS autoimmunity, we used mice deficient for copper-zinc superoxide dismutase (SOD1), an endogenous regulator of ROS. SOD1-deficient C57Bl/6 mice were found to develop more severe EAE induced with MOG 35-55 as compared with their littermate wild type controls. This alteration in the disease phenotype was not due to aberrant expansion of MOG-specific T cells nor their ability to produce inflammatory cytokines; rather, lymphocytes generated in SOD1-deficient mice were more prone to spontaneous cell death when compared with their wild type littermate controls. The data point to a role for SOD1 in the maintenance of self-tolerance leading to the suppression of autoimmune responses.
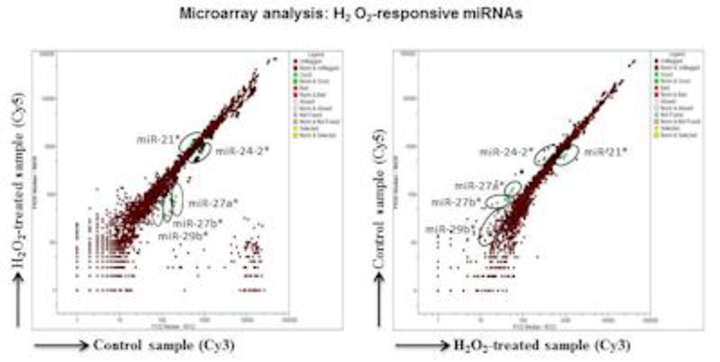
In addition, to dissect the complex nature of ROS-mediated effects in immune cells, we made efforts to characterize microRNAs (miRNAs) that are responsive to oxidative stress, leading us to identify a set of miRNAs to be uniquely expressed in response to hydrogen peroxide in the mouse macrophage cell line, RAW 264.7. One of these, miR-27b*, has a modulating effect on nuclear factor-kB activation, suggesting a role for oxidative stress-responsive miRNAs in modulating the innate immune functions.
Urinary metabolites as biomarkers of CNS autoimmunity
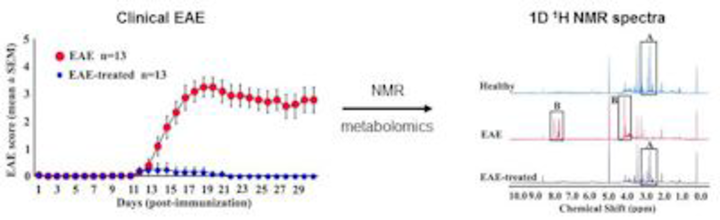
Using fingolimod as a disease-modifying agent in MOG 35-55-induced EAE, we made efforts to identify the urinary metabolites that are indicative of disease progression and to monitor response to therapy. NMR analysis of metabolites allowed us to distinguish EAE from healthy mice and from those treated with or without fingolimod, suggesting that urinary metabolites have significant promise for assessing CNS autoimmunity. Efforts are under way to investigate the urinary metabolites in a clinical cohort study involving patients with MS and other neurological diseases.
Additional Information
Potential of Urinary Metabolites for Diagnosing Multiple Sclerosis
Uveitis/uveoretinitis
Experimental autoimmune uveoretinitis (EAU)
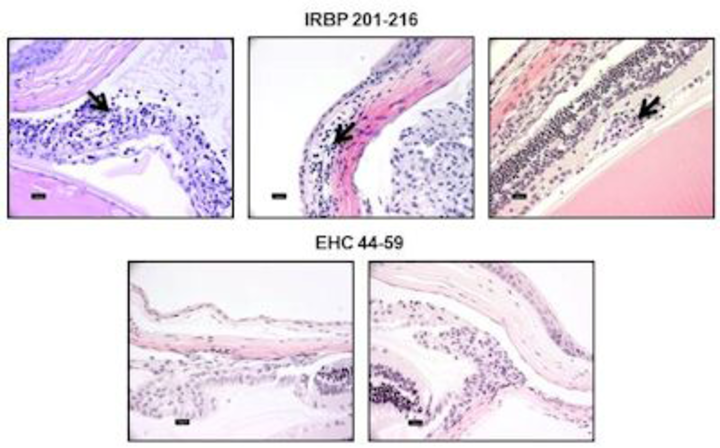
To identify the infectious triggers of uveitis and their underlying mechanisms, we established an EAU model induced with interphotoreceptor retinoid-binding protein (IRBP) 201-216 in A/J mice. Our focus is to identify pathogens primarily affecting the eyes with an expectation that such microbes can serve as ideal candidates to delineate the autoimmune events of uveitic conditions. We discovered that although one mimicry epitope derived from Ehrlichia canis could induce IRBP-specific T cells, it failed to induce EAU. Rather, the mimicry epitope prevented the disease induced with IRBP 201-216 by acting as a naturally occurring altered peptide ligand. We theorize that in typical host-parasite relationships, microbes carrying mimicry epitopes can induce pathogenic cross-reactive immune responses, which may result in the death of the host. Such outcomes are not favorable for survival of parasites. Alternatively, from an evolutionary point of view, it may be possible that as a parasite coexists with a host, it can acquire the host’s genetic material; introduction of mutations under selection pressure, if any, may favor attenuation of the host’s immune response, leading to the parasite’s survival.